Summary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Product has been found to contain yeast/mold and microbial contamination with one species identified as Achromobacter, at levels above specifications.
- Company Name:
- MediNatura New Mexico Inc.
- Brand Name:
- Product Description:
Company Announcement
December 10, 2025 – Albuquerque, New Mexico, MediNatura New Mexico, Inc. is voluntarily recalling one lot of ReBoost Nasal Spray to the consumer level. The product has been found to contain yeast/mold and microbial contamination with one species identified as Achromobacter, at levels above specifications.
Risk Statement: There is a reasonable probability that adverse health consequences including life-threatening infections will occur with use of the product in the immuno-compromised population. To date, MediNatura has not received any reports of adverse events related to this recall.
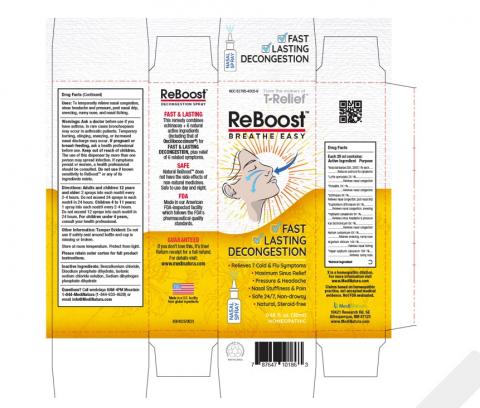
The product is used as a homeopathic nasal spray to temporarily relieve nasal congestion, sinus headache and pressure, postnasal drip, sneezing, runny nose, and nasal itching and is packaged in a 20mL bottle which is further packaged in a white and yellow carton. The NDC number is 62795-4005-9 and the UPC# is 787647 10186 3. The recalled ReBoost product includes the following lot number 224268 with an expiration date of 12/2027. The product can be identified by its bottle label and carton:
ReBoost was distributed nationwide via retail and internet sales(medinatura.com).
All customers should immediately discontinue using the ReBoost under recall. Customers who purchased the product directly from MediNatura New Mexico, Inc. should contact MediNatura New Mexico, Inc. at recall@medinatura.com to arrange for a refund. Consumers who purchased the product at retailers should return it to the place of purchase.
Consumers with questions regarding this recall can contact MediNatura New Mexico, Inc by phone (800-621-7644) or recall@medinatura.com; Monday-Friday, 8:00 AM to 5:00 PM MST.Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product. Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
Product Photos
Credit: Source link